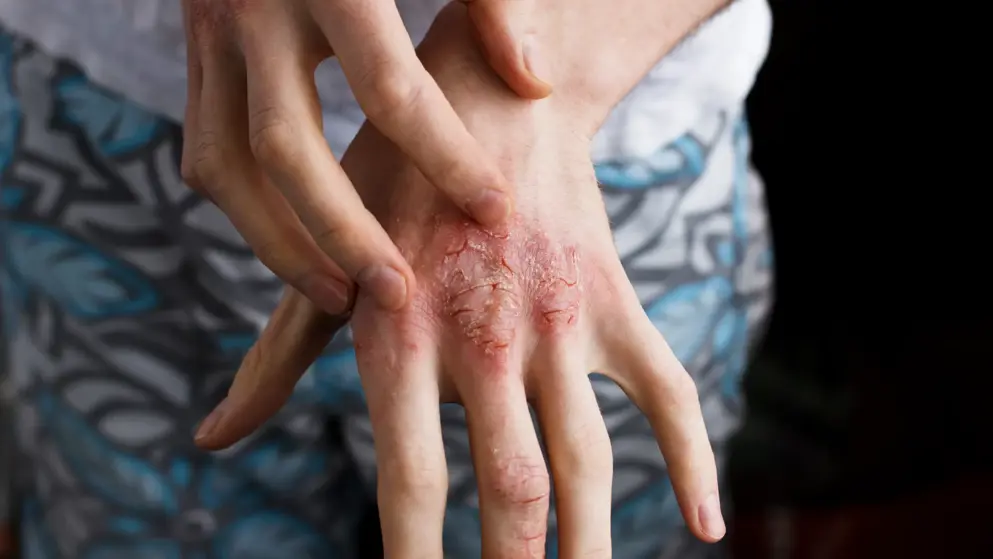
Dupixent data at AAD show significant improvements in signs and symptoms of moderate-to-severe atopic hand and foot dermatitis.- Regeneron + Sanofi
Regeneron Pharmaceuticals, Inc. and Sanofi presented positive results from the clinical trial assessing Dupixent (dupilumab) in adults and adolescents with uncontrolled moderate-to-severe atopic hand and foot dermatitis.
The trial, the first evaluating a biologic for this difficult-to-treat population, met its primary and key secondary endpoints. The results were featured in a late-breaking session, one of more than 20 Dupixent scientific presentations, at the American Academy of Dermatology (AAD) 2023 Annual Meeting.
“Atopic hand and foot dermatitis can extensively disrupt the lives of patients, given the intense itch and painful skin lesions it causes on essential body areas,” said Eric L. Simpson, M.D., Frances J. Storrs Professor of Medical Dermatology at the Oregon Health and Science University and principal investigator of this trial. “In this trial, Dupixent significantly improved disease signs, symptoms and quality of life measures for this particularly difficult-to-treat subset of atopic dermatitis patients, with itch improvement seen as early as one week after the first dose. While the efficacy and safety profile of Dupixent is well-established for atopic dermatitis more broadly, these positive results are the first demonstrating the impact on specific and heavily used areas of the body.”
In the trial, patients received Dupixent (n=67) every two weeks (adults 300 mg, adolescents 200 mg or 300 mg based on body weight) or placebo (n=66). At 16 weeks, patients treated with Dupixent experienced the following : i. 40% achieved clear or almost clear skin on hands and feet compared to 17% with placebo (p-<0.01), the primary endpoint. ii. 52% saw a clinically meaningful reduction in itch on hands and feet compared to 14% with placebo (p><0.0001), the key secondary endpoint. iii. 69% average reduction in signs of hand and foot lesions from baseline compared to 31% with placebo (p><0.0001). iv. 75% average improvement in hand eczema disease severity from baseline compared to 40% with placebo (p><0.0001). v. there were significant improvements in measures of hand and foot skin pain, sleep, and hand eczema-related quality of life.></0.0001).></0.0001).></0.0001),></0.01),>
The trial demonstrated similar safety results to the known safety profile of Dupixent in atopic dermatitis. Overall rates of adverse events (AEs) were 66% for Dupixent and 74% for placebo. AEs more commonly observed with Dupixent (greater than 5%) compared to placebo included nasopharyngitis (16% Dupixent, 11% placebo), upper respiratory tract infection (9% Dupixent, 5% placebo), conjunctivitis (6% Dupixent, 2% placebo), herpes viral infections (6% Dupixent, 3% placebo) and increased blood creatine phosphokinase (6% Dupixent, 0% placebo). Additionally, 3% of patients taking Dupixent used at least one rescue medication compared to 21% of patients on placebo.
There are 23 Dupixent scientific abstracts being presented across three dermatological diseases with underlying type 2 inflammation at the AAD 2023 Annual Meeting. These include oral presentations on long-term Dupixent use in children as young as 6 months with atopic dermatitis; the impact of Dupixent treatment on health-related quality of life, skin pain and sleep in prurigo nodularis; and the investigational use of Dupixent on signs, symptoms and health-related quality of life in chronic spontaneous urticaria.
About the Dupixent Trial The Phase III double-blind, placebo-controlled trial evaluated the efficacy and safety of Dupixent in 133 adolescents and adults with moderate-to-severe atopic hand and foot dermatitis who had an inadequate response or intolerance to topical corticosteroids. Patients with hand and foot disease predominantly driven by allergic or irritant contact dermatitis were excluded from the trial. The primary endpoint evaluated the proportion of patients with clear or almost clear skin of hand and feet eczema at 16 weeks (measured by a score of 0 or 1 on the Investigator Global Assessment Scale). The key secondary endpoint measured the proportion of patients with improvement in itch on hands and feet from baseline (measured by a greater than 4-point reduction in Peak-Pruritis Numeric Rating Scale [PP-NRS] on a 0-10 scale) at 16 weeks. Lesion sign reduction was assessed by change from baseline in Modified Total Lesion Sign Score (mTLSS; measured by a 0-36 scale), and disease severity was assessed by the change from baseline in Hand Eczema Severity Index (HECSI) score (measured by a 0-360 scale). Symptoms were assessed every one or two weeks during the trial.
Additional secondary endpoints included ; i. Skin pain reduction as assessed by the change from baseline in weekly average of daily hand and foot peak pain NRS (measured by a 0-10 scale). ii. Sleep improvement as assessed by change from baseline in weekly average of daily Sleep NRS (measured by a 0-10 scale). iii. Health-related quality of life, assessed by change from baseline in the Quality of Life in Hand Eczema Questionnaire (QoLHEQ) (measured by a 0-117 scale).