Lilly's Ebglyss (lebrikizumab-lbkz) demonstrated meaningful improvement in skin clearance and itch relief in the majority of patients with moderate-to-severe atopic dermatitis who discontinued dupilumab- Eli Lilly
New results show Eli Lilly's Ebglyss improved skin (including hand and face) and itch among patients with moderate-to-severe atopic dermatitis (eczema) who were previously treated with dupilumab.
These results from the Phase IIIb ADapt study will be presented at the Fall Clinical Dermatology (FCD) Conference from Oct. 24-27 in Las Vegas. ADapt (NCT05369403), is an open-label, 24-week study that evaluated the efficacy and safety of Ebglyss in adults and adolescents (12 to less than 18 years of age and weighing ≥40 kg) with moderate-to-severe atopic dermatitis who were previously treated with dupilumab. Four or more weeks after discontinuing dupilumab, patients were treated with EBGLYSS and received a starting dosing of 500 mg (two 250 mg injections) at Week 0 and Week 2, followed by 250 mg every two weeks until Week 16. IGA 0,1 or EASI-75 responders at Week 16 received 250 mg once monthly and non-responders continued on 250 mg every two weeks until Week 24. Patients were allowed to stay on low and mid-potency topical corticosteroids. From baseline to Week 16, data from the ADapt study was analyzed as observed and with non-responder imputation/multiple imputation (NRI/MI). After Week 16, Q2W and Q4W data from the ADapt study were pooled and analyzed as observed and with NRI/MI.
The ADapt study evaluated the efficacy and safety of Ebglyss n patients with moderate-to-severe atopic dermatitis who were previously treated with dupilumab. To qualify for ADapt, patients must have discontinued dupilumab treatment due to inadequate response, intolerance or an adverse event, or other reasons (including cost or loss of access to the medicine). The primary endpoint of the study was measured by at least 75 percent improvement in the Eczema Area and Severity Index (EASI-75) score at 16 weeks, which evaluates the extent and severity of the skin disease. Secondary endpoints at 16 and 24 weeks included Investigator Global Assessment (IGA) score of clear (0) or almost clear (1) skin with a reduction of at least two points from baseline and at least a four-point improvement in Pruritus NRS from baseline. Other secondary and exploratory endpoints were also included. The reported endpoints were as observed.
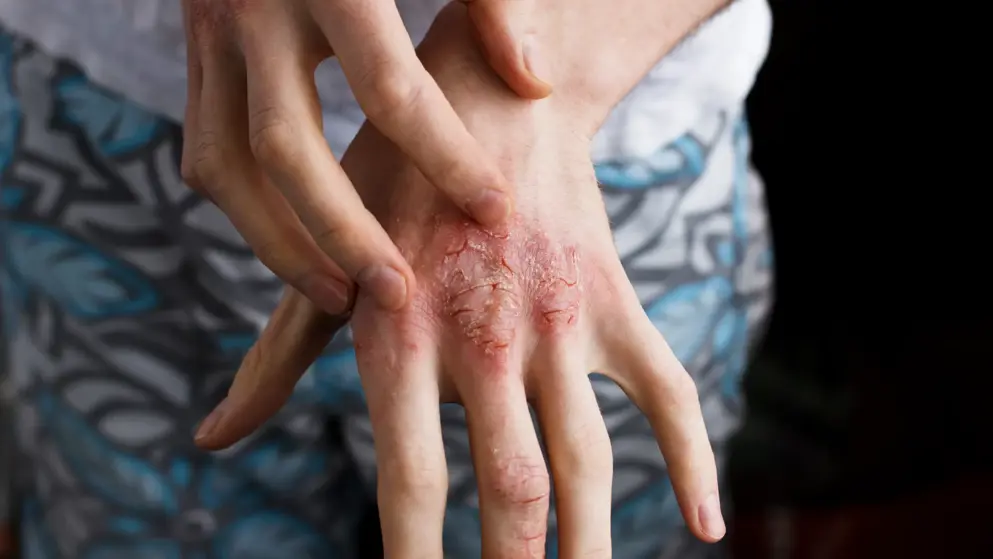
With Ebglyss 57 percent of patients at Week 16, and 60 percent of patients at Week 24 who were previously treated with dupilumab, achieved EASI-75. These results are similar to what was observed in the Phase III monotherapy trials of Ebglyss in patients without prior exposure to dupilumab (ADvocate 1 and ADvocate 2). In addition, 46 percent of patients who were inadequate responders to dupilumab achieved EASI-75 response with Ebglyss at Week 16. Of the ADapt patients who discontinued dupilumab and began treatment with Ebglyss: 53 percent and 62 percent also experienced itch relief (Pruritus NRS) with at least a four-point improvement from baseline at Week 16 and Week 24 respectively. Patients in this study saw improvements in difficult-to-treat areas when treated with Ebglyss. More than half of patients (52 percent) treated with Ebglyss saw clear or almost clear face dermatitis at Week 24 (F-IGA 0,1 with a reduction of at least two points from baseline). Among patients with moderate-to-severe hand dermatitis at baseline (defined as ≥12), patients' modified total lesion symptom score (mTLSS), which measures extent and severity of hand dermatitis, decreased by 75 percent at Week 24.
Less than 6 percent of patients treated with Ebglyss experienced an adverse event that led to treatment discontinuation. The safety profile of Ebglyss in ADapt was consistent with previous Ebglyss Phase III studies in patients with moderate-to-severe atopic dermatitis, and no new safety signals were observed. The majority of adverse events were mild or moderate. Reported treatment-related side effects in the study were conjunctivitis and injection site reactions. Of the 14 patients who discontinued dupilumab due to an adverse event, two patients discontinued Ebglyss due to an adverse event. Of the 10 patients who discontinued dupilumab due to eye-related events, facial dermatitis or inflammatory arthritis, none reported similar events with Ebglyss.
Lilly will also present additional data at the Fall (autumn) Clinical Dermatology conference, including new analyses from the ADjoin long-term extension study with data up to three years.
"This trial supports the growing body of data showing that health care providers can have confidence prescribing EBGLYSS as a first-line biologic treatment for moderate-to-severe atopic dermatitis, and reinforces that EBGLYSS provided a meaningful benefit among individuals who have already tried another biologic treatment such as dupilumab and may have more difficult-to-treat disease," said Dr. Mark Genovese, SVPt of Lilly Immunology development.
"Treatment isn't one-size-fits-all, and many patients with moderate-to-severe atopic dermatitis remain in need of an effective medicine to help manage the impact of the disease, especially in difficult-to-treat areas like face and hands," said Dr. Linda Stein Gold, investigator of the ADapt study, director of dermatology research and head of the Division of Dermatology for Henry Ford Health System in Detroit, Michigan. "These data showed that EBGLYSS improved skin symptoms and reduced itch for the majority of patients who had stopped using dupilumab and complement previously presented EBGLYSS data in biologic-naive patients, further supporting that a broad range of patients could benefit from this new and effective treatment option."