J&J licenses STAT6 program for autoimmune diseases
Johnson & Johnson announced that it has entered into an exclusive licensing agreement for the global development, manufacturing and commercialization of a STAT6 program for autoimmune and allergic diseases, including atopic dermatitis (AD), from Kaken Pharmaceutical
Johnson & Johnson gains an exclusive license to Kaken’s STAT6 program, including lead candidate KP 723. Kaken will retain the commercialization rights in Japan, where Johnson & Johnson will have an option to enter into a co-promotion agreement with Kaken. Separately, Kaken is eligible to receive an equity investment from Johnson & Johnson Innovation – JJDC, Inc., the venture capital organization of Johnson & Johnson.
By modulating STAT6, KP 723 complements the Company’s portfolio of molecules targeting pathways relevant to AD. KP 723 is anticipated to begin Phase 1 trials in AD next year and may have applications in other Th2-mediated diseases, including asthma.
“To address the significant unmet need for people living with the highly heterogeneous diseases of atopic dermatitis and asthma, we must have multiple treatment candidates in our pipeline that target key pathways driving disease progression,” said David Lee, Global Immunology Therapeutic Area Head, Johnson & Johnson Innovative Medicine. “STAT6 represents a promising area of research, as it offers a potential for an effective and safe oral option for people struggling to manage atopic dermatitis and other autoimmune diseases.”
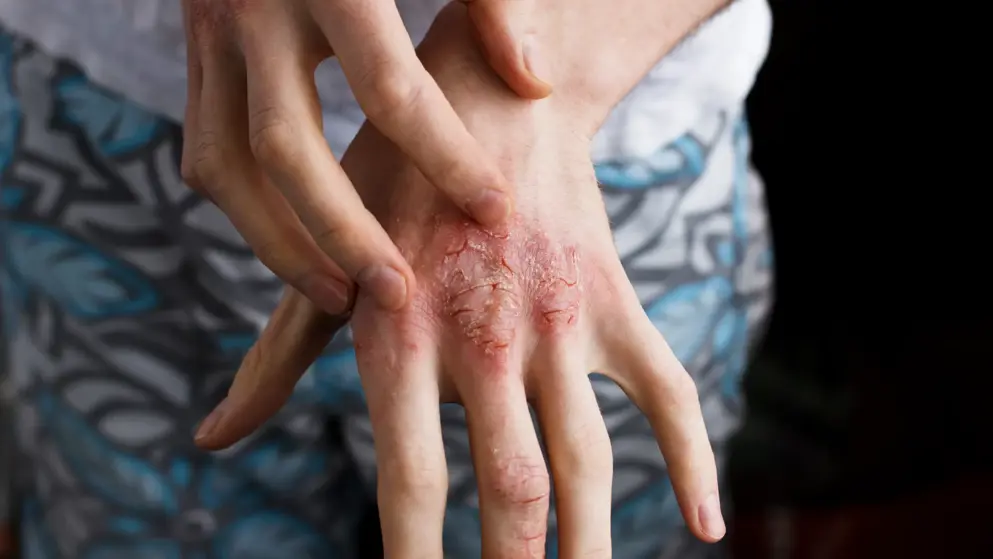
AD is the most common inflammatory skin disease. It causes itching and inflammation, which are made worse by scratching. It can lead to increased risk of skin infections, skin pain, difficulty sleeping, anxiety, stress, depression and even an increased risk of suicide.
“Nearly three-quarters of people with atopic dermatitis are not achieving remission with currently available treatments,” said Candice Long, Worldwide Vice President, Immunology, Johnson & Johnson. “Our investment in KP 723 may allow us to provide a novel treatment option with the convenience of a pill for patients living with this complex immune-mediated disease.”